
"It is JUST AS surprising - as if a gunner fired a shell at a single sheet of paper and for some reason or other, the projectile BOUNCED BACK."
This is how New Zealand physicist Ernest Rutherford described the result of alpha particle scattering experiment - conducted by his students Geiger and Marsden.
Introduction
Geiger and Marsden aimed high speed alpha particles at a very thin gold foil - it was only 1000 atoms thick. Around the gold foil was a zinc sulphide screen which glowed every time alpha particles would hit it.
If Thomson's plum pudding model of atom were correct, the fast moving and relatively heavier alpha particles would have passed straight thought the target, since electric field generated by evenly distributed charge is very minimal.
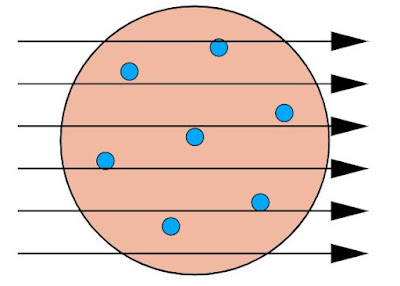
But the experiment revealed that a few alpha particles were deflected by small angles, while 1 in 20,000 particles got deviated by angle greater than 90 degrees.
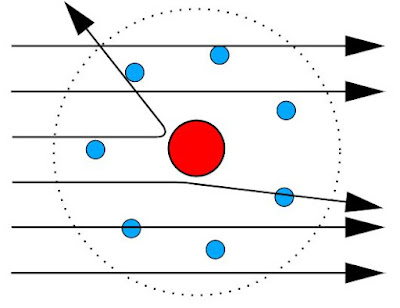
Rutherford set out to explain these unusual findings by creating a new model of atom, because Thomson's model had failed.
Early life and career
Ernest Rutherford [1871-1937] was a multi-talented student who did phenomenally well in mathematics, catching everyone's attention at his school as a consequence.
He won the scholarship to study at Canterbury College, University of New Zealand, where he participated not only in the lab but also in the debating society.
Rutherford was the head boy in college and played the rugged sport of rugby. He completed three degrees in this college - ba, ma and bsc.
Thereafter, he travelled to England in order to study under the guidance of J. J. Thomson at the Cambridge University. Rutherford worked with cathode ray tubes under Thomson's mentorship.
In 1899, he heard about Henri Becquerel's discovery of radioactivity and became interested in exploring alpha and beta decay. Rutherford was among the first to prove that alpha particles were Helium nuclei.
"All science is either physics or stamp collecting." Rutherford used to say, but ironically he won the Nobel Prize in chemistry in 1908 for his pioneering work with on the chemistry of radioactive substances.
Discovery of Nucleus
As discussed earlier, alpha particle scattering experiment was conducted by Rutherford, Geiger and Marsden in the year 1909, by passing alpha particles through a
thin gold foil.
Rutherford argued that since most of the particles passed
straight through the gold foil, the atom must be made up of mostly empty space - not a positive soup as Thomson had thought.
In fact, the atom is about 100,000 times the diameter of the nucleus. It is like putting a grain of sand in the middle of a soccer ground!
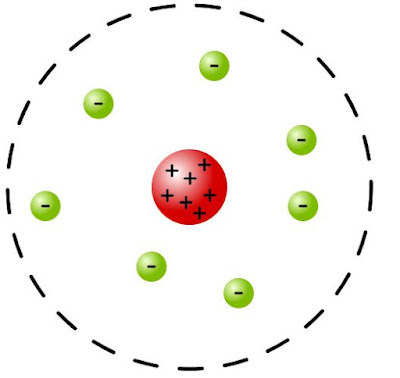
Positive charge must be localized, Rutherford argued, in a very small point at the center of atom, which explained bouncing back in a small fraction of alpha particles, since positive positive repel.
Negative charges in the atom must be located somewhere on the outskirts ... which
explained smaller deflections.
Summing up
By creating a new improved model, Rutherford became the father of nuclear physics, as he initiated a whole new branch of physics. Scientists decided to probe further into the nucleus and many subatomic particles were discovered as a result.
Upon the discovery of atomic nucleus, Rutherford said: "I have broken the machine and touched the GHOST OF MATTER." But he regretted not being able to explain something deeper - "when we found the nucleus, we found the basis of everything, the greatest secret of all - except of life."






 Physics, astronomy and science history blog for students
Physics, astronomy and science history blog for students
Responsive Ad Slot